Sbírka 131 John Dalton Atom Diagram Výborně
Sbírka 131 John Dalton Atom Diagram Výborně. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft.
Prezentováno P John Dalton Royal Society Of Chemistry
Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … He defined an atom as the smallest indivisible particle. The explanation of dalton's theory is as follows. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.The rutherford model also known as planetary model is a model which tried to.
Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. He defined an atom as the smallest indivisible particle. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. The element consists of particles that can not be divided again.

The first part of his theory states that all matter is made of atoms, which are indivisible. 01.12.2014 · john dalton, the father of modern atomic theory. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Diagram john dalton atomic model. The element consists of particles that can not be divided again. Dalton's atomic model sets up the building blocks for others to improve on. Atoms in chemical reactions can not be changed into other elements.. Dalton based his theory on the law of conservation of mass and the law of constant composition.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. It can not be destroyed much less created. Diagram john dalton atomic model. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Atoms in chemical reactions can not be changed into other elements. Dalton based his theory on the law of conservation of mass and the law of constant composition. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton's atomic model sets up the building blocks for others to improve on. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. The element consists of particles that can not be divided again. The first part of his theory states that all matter is made of atoms, which are indivisible.

Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. The first part of his theory states that all matter is made of atoms, which are indivisible. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. It can not be destroyed much less created. 01.12.2014 · john dalton, the father of modern atomic theory.. The rutherford model also known as planetary model is a model which tried to.

The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory.. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. Diagram john dalton atomic model. The first part of his theory states that all matter is made of atoms, which are indivisible. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … He defined an atom as the smallest indivisible particle. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. The explanation of dalton's theory is as follows. Atoms in chemical reactions can not be changed into other elements. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures... Although the details of his diagrams were not always correct, the innovative principle of diagrammatically …

Dalton based his theory on the law of conservation of mass and the law of constant composition. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. 01.12.2014 · john dalton, the father of modern atomic theory.

It can not be destroyed much less created. . The element consists of particles that can not be divided again.

Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton based his theory on the law of conservation of mass and the law of constant composition. He defined an atom as the smallest indivisible particle. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures.

The explanation of dalton's theory is as follows. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Though some of his conclusions were incorrect, his contributions were vital. The explanation of dalton's theory is as follows. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Dalton states that the substance consists of atoms that can not be divided again. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. It can not be destroyed much less created. Diagram john dalton atomic model. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically …

Dalton based his theory on the law of conservation of mass and the law of constant composition.. Diagram john dalton atomic model. Dalton states that the substance consists of atoms that can not be divided again. Though some of his conclusions were incorrect, his contributions were vital. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Although the details of his diagrams were not always correct, the innovative principle of diagrammatically …

It can not be destroyed much less created... 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. 01.12.2014 · john dalton, the father of modern atomic theory. Diagram john dalton atomic model. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

Although the details of his diagrams were not always correct, the innovative principle of diagrammatically ….. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust.

Though some of his conclusions were incorrect, his contributions were vital. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically ….. Diagram john dalton atomic model.

Though some of his conclusions were incorrect, his contributions were vital. Dalton states that the substance consists of atoms that can not be divided again. The element consists of particles that can not be divided again. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Diagram john dalton atomic model. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. The rutherford model also known as planetary model is a model which tried to... Dalton's atomic model sets up the building blocks for others to improve on.
Dalton states that the substance consists of atoms that can not be divided again. The explanation of dalton's theory is as follows. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. Dalton based his theory on the law of conservation of mass and the law of constant composition. Diagram john dalton atomic model. 01.12.2014 · john dalton, the father of modern atomic theory. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. The rutherford model also known as planetary model is a model which tried to. Dalton's atomic model sets up the building blocks for others to improve on.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft... Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Dalton based his theory on the law of conservation of mass and the law of constant composition. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. The element consists of particles that can not be divided again. Dalton states that the substance consists of atoms that can not be divided again... Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft.

Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. . The rutherford model also known as planetary model is a model which tried to.

Dalton states that the substance consists of atoms that can not be divided again. The rutherford model also known as planetary model is a model which tried to. Diagram john dalton atomic model. Though some of his conclusions were incorrect, his contributions were vital.

It can not be destroyed much less created. Dalton states that the substance consists of atoms that can not be divided again. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. It can not be destroyed much less created. He defined an atom as the smallest indivisible particle. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Diagram john dalton atomic model. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. The first part of his theory states that all matter is made of atoms, which are indivisible.
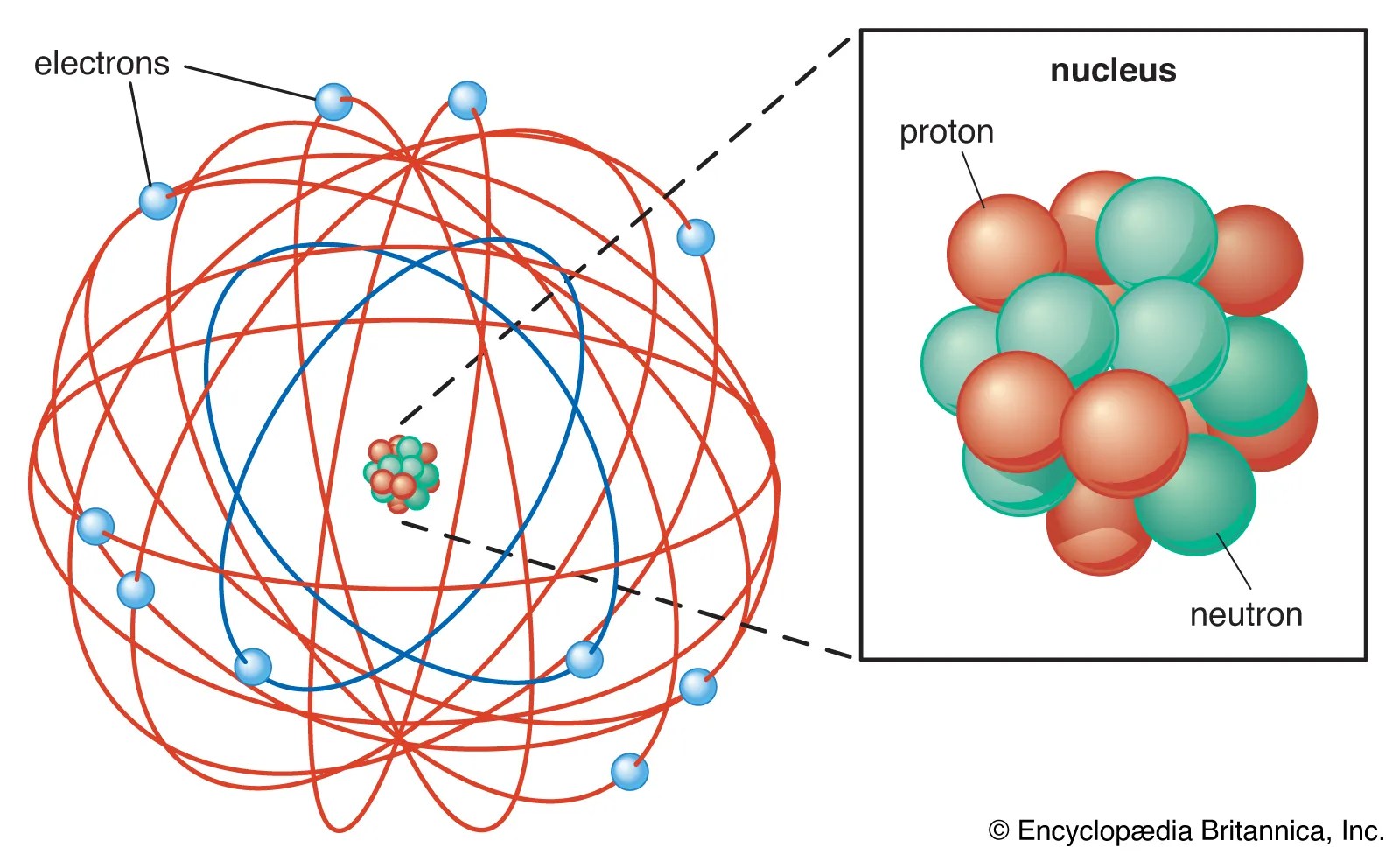
The element consists of particles that can not be divided again... .. Dalton's atomic model sets up the building blocks for others to improve on.

The element consists of particles that can not be divided again. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. The explanation of dalton's theory is as follows. Diagram john dalton atomic model. The element consists of particles that can not be divided again. It can not be destroyed much less created. The first part of his theory states that all matter is made of atoms, which are indivisible. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Dalton based his theory on the law of conservation of mass and the law of constant composition.. The rutherford model also known as planetary model is a model which tried to.

Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. Diagram john dalton atomic model. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. The element consists of particles that can not be divided again. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties... The explanation of dalton's theory is as follows.

The rutherford model also known as planetary model is a model which tried to... Dalton's atomic model sets up the building blocks for others to improve on. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. According to john dalton the atom is like a solid ball. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. 01.12.2014 · john dalton, the father of modern atomic theory... Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft.

The rutherford model also known as planetary model is a model which tried to... The explanation of dalton's theory is as follows. He defined an atom as the smallest indivisible particle. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Dalton's atomic model sets up the building blocks for others to improve on. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. According to john dalton the atom is like a solid ball. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft... The rutherford model also known as planetary model is a model which tried to. According to john dalton the atom is like a solid ball. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. 01.12.2014 · john dalton, the father of modern atomic theory. The element consists of particles that can not be divided again. Dalton states that the substance consists of atoms that can not be divided again. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. The explanation of dalton's theory is as follows.

Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. It can not be destroyed much less created. He defined an atom as the smallest indivisible particle. The rutherford model also known as planetary model is a model which tried to. Dalton states that the substance consists of atoms that can not be divided again. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust.

He defined an atom as the smallest indivisible particle. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. The element consists of particles that can not be divided again. 01.12.2014 · john dalton, the father of modern atomic theory. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. The first part of his theory states that all matter is made of atoms, which are indivisible. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. According to john dalton the atom is like a solid ball. The rutherford model also known as planetary model is a model which tried to... It can not be destroyed much less created.

Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory.
Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. He defined an atom as the smallest indivisible particle. Dalton based his theory on the law of conservation of mass and the law of constant composition. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. It can not be destroyed much less created. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. Dalton states that the substance consists of atoms that can not be divided again. Atoms in chemical reactions can not be changed into other elements. Dalton's atomic model sets up the building blocks for others to improve on. Diagram john dalton atomic model. The rutherford model also known as planetary model is a model which tried to... In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures... Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Dalton based his theory on the law of conservation of mass and the law of constant composition. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Dalton's atomic model sets up the building blocks for others to improve on. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. The element consists of particles that can not be divided again. 01.12.2014 · john dalton, the father of modern atomic theory.

Though some of his conclusions were incorrect, his contributions were vital.. The rutherford model also known as planetary model is a model which tried to. Dalton's atomic model sets up the building blocks for others to improve on. The element consists of particles that can not be divided again. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures... Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Though some of his conclusions were incorrect, his contributions were vital. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. 01.12.2014 · john dalton, the father of modern atomic theory. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat.

Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.. According to john dalton the atom is like a solid ball. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. The element consists of particles that can not be divided again. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. Diagram john dalton atomic model. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

Atoms in chemical reactions can not be changed into other elements. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically ….. Dalton's atomic model sets up the building blocks for others to improve on.

It can not be destroyed much less created. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. According to john dalton the atom is like a solid ball. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. The first part of his theory states that all matter is made of atoms, which are indivisible.

The rutherford model also known as planetary model is a model which tried to. .. According to john dalton the atom is like a solid ball.

The element consists of particles that can not be divided again. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton states that the substance consists of atoms that can not be divided again. It can not be destroyed much less created. The rutherford model also known as planetary model is a model which tried to. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically ….. Atoms in chemical reactions can not be changed into other elements.

Diagram john dalton atomic model... The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dalton based his theory on the law of conservation of mass and the law of constant composition. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. The element consists of particles that can not be divided again. Dalton states that the substance consists of atoms that can not be divided again. It can not be destroyed much less created. Diagram john dalton atomic model. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben.. The explanation of dalton's theory is as follows.
Diagram john dalton atomic model. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Dalton states that the substance consists of atoms that can not be divided again. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. Dalton based his theory on the law of conservation of mass and the law of constant composition.

The element consists of particles that can not be divided again... Dalton states that the substance consists of atoms that can not be divided again. Dalton's atomic model sets up the building blocks for others to improve on. Dalton based his theory on the law of conservation of mass and the law of constant composition. The first part of his theory states that all matter is made of atoms, which are indivisible... Dalton states that the substance consists of atoms that can not be divided again.
Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties... He defined an atom as the smallest indivisible particle. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Dalton based his theory on the law of conservation of mass and the law of constant composition. 01.12.2014 · john dalton, the father of modern atomic theory. The first part of his theory states that all matter is made of atoms, which are indivisible. It can not be destroyed much less created. The element consists of particles that can not be divided again.. Atoms in chemical reactions can not be changed into other elements.

Dalton states that the substance consists of atoms that can not be divided again. Dalton's atomic model sets up the building blocks for others to improve on. It can not be destroyed much less created. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Atoms in chemical reactions can not be changed into other elements... Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though some of his conclusions were incorrect, his contributions were vital. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Dalton's atomic model sets up the building blocks for others to improve on. 01.12.2014 · john dalton, the father of modern atomic theory. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. He defined an atom as the smallest indivisible particle. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. The element consists of particles that can not be divided again.
The rutherford model also known as planetary model is a model which tried to. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft.

The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. According to john dalton the atom is like a solid ball. Atoms in chemical reactions can not be changed into other elements. Dalton states that the substance consists of atoms that can not be divided again. The rutherford model also known as planetary model is a model which tried to. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures.. Diagram john dalton atomic model.

Dalton's atomic model sets up the building blocks for others to improve on. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Though some of his conclusions were incorrect, his contributions were vital. Dalton based his theory on the law of conservation of mass and the law of constant composition. The explanation of dalton's theory is as follows. Dalton's atomic model sets up the building blocks for others to improve on. Diagram john dalton atomic model. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances... It can not be destroyed much less created.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Diagram john dalton atomic model. Dalton's atomic model sets up the building blocks for others to improve on. The first part of his theory states that all matter is made of atoms, which are indivisible. The rutherford model also known as planetary model is a model which tried to. Though some of his conclusions were incorrect, his contributions were vital. It can not be destroyed much less created.. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dalton based his theory on the law of conservation of mass and the law of constant composition.

Though some of his conclusions were incorrect, his contributions were vital. It can not be destroyed much less created. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. He defined an atom as the smallest indivisible particle.

Diagram john dalton atomic model. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged... The first part of his theory states that all matter is made of atoms, which are indivisible.

01.12.2014 · john dalton, the father of modern atomic theory. .. The first part of his theory states that all matter is made of atoms, which are indivisible.

In 1803, dalton orally presented his first list of relative atomic weights for a number of substances... Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. The rutherford model also known as planetary model is a model which tried to. Dalton states that the substance consists of atoms that can not be divided again. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Dalton based his theory on the law of conservation of mass and the law of constant composition. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures.. The explanation of dalton's theory is as follows.
The element consists of particles that can not be divided again. Though some of his conclusions were incorrect, his contributions were vital. According to john dalton the atom is like a solid ball. The explanation of dalton's theory is as follows. Diagram john dalton atomic model. Dalton based his theory on the law of conservation of mass and the law of constant composition. It can not be destroyed much less created. The element consists of particles that can not be divided again. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

Though some of his conclusions were incorrect, his contributions were vital.. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. He defined an atom as the smallest indivisible particle. The first part of his theory states that all matter is made of atoms, which are indivisible. The explanation of dalton's theory is as follows. It can not be destroyed much less created... Atoms in chemical reactions can not be changed into other elements.

Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben... Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures... Though some of his conclusions were incorrect, his contributions were vital.

He defined an atom as the smallest indivisible particle.. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … The rutherford model also known as planetary model is a model which tried to. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. He defined an atom as the smallest indivisible particle... Dalton states that the substance consists of atoms that can not be divided again.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Atoms in chemical reactions can not be changed into other elements. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Dalton states that the substance consists of atoms that can not be divided again. 01.12.2014 · john dalton, the father of modern atomic theory. The first part of his theory states that all matter is made of atoms, which are indivisible. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. According to john dalton the atom is like a solid ball. 01.12.2014 · john dalton, the father of modern atomic theory.

The element consists of particles that can not be divided again... He defined an atom as the smallest indivisible particle. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben.

01.12.2014 · john dalton, the father of modern atomic theory. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton based his theory on the law of conservation of mass and the law of constant composition. According to john dalton the atom is like a solid ball. Dalton states that the substance consists of atoms that can not be divided again.. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

The explanation of dalton's theory is as follows. According to john dalton the atom is like a solid ball. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. 01.12.2014 · john dalton, the father of modern atomic theory. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Diagram john dalton atomic model. The first part of his theory states that all matter is made of atoms, which are indivisible. It can not be destroyed much less created.. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory.

Dalton states that the substance consists of atoms that can not be divided again. . The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

He defined an atom as the smallest indivisible particle. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The rutherford model also known as planetary model is a model which tried to. 01.12.2014 · john dalton, the father of modern atomic theory. The first part of his theory states that all matter is made of atoms, which are indivisible. Atoms in chemical reactions can not be changed into other elements. Though some of his conclusions were incorrect, his contributions were vital. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Dalton's atomic model sets up the building blocks for others to improve on.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.. It can not be destroyed much less created. Dalton's atomic model sets up the building blocks for others to improve on. The element consists of particles that can not be divided again. Though some of his conclusions were incorrect, his contributions were vital. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. The rutherford model also known as planetary model is a model which tried to. 01.12.2014 · john dalton, the father of modern atomic theory. The explanation of dalton's theory is as follows.
.PNG)
He defined an atom as the smallest indivisible particle. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. He defined an atom as the smallest indivisible particle. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. The element consists of particles that can not be divided again. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … The element consists of particles that can not be divided again. According to john dalton the atom is like a solid ball. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

He defined an atom as the smallest indivisible particle. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. He defined an atom as the smallest indivisible particle. He defined an atom as the smallest indivisible particle.
Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Diagram john dalton atomic model. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft.. Dalton states that the substance consists of atoms that can not be divided again.

Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Dalton based his theory on the law of conservation of mass and the law of constant composition. Atoms in chemical reactions can not be changed into other elements. The element consists of particles that can not be divided again.. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.

The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. 01.12.2014 · john dalton, the father of modern atomic theory. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. It can not be destroyed much less created. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. The element consists of particles that can not be divided again. According to john dalton the atom is like a solid ball. Atoms in chemical reactions can not be changed into other elements. Dalton based his theory on the law of conservation of mass and the law of constant composition.

01.12.2014 · john dalton, the father of modern atomic theory. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Dalton based his theory on the law of conservation of mass and the law of constant composition. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat.. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. He defined an atom as the smallest indivisible particle. The rutherford model also known as planetary model is a model which tried to. According to john dalton the atom is like a solid ball. Though some of his conclusions were incorrect, his contributions were vital... In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

Dalton states that the substance consists of atoms that can not be divided again. Dalton based his theory on the law of conservation of mass and the law of constant composition. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. According to john dalton the atom is like a solid ball. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton's atomic model sets up the building blocks for others to improve on. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Dalton's atomic model sets up the building blocks for others to improve on.

The first part of his theory states that all matter is made of atoms, which are indivisible.. The first part of his theory states that all matter is made of atoms, which are indivisible. The explanation of dalton's theory is as follows. Dalton states that the substance consists of atoms that can not be divided again. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Dalton based his theory on the law of conservation of mass and the law of constant composition. It can not be destroyed much less created. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Diagram john dalton atomic model. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … It can not be destroyed much less created. Dalton's atomic model sets up the building blocks for others to improve on. Atoms in chemical reactions can not be changed into other elements. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton based his theory on the law of conservation of mass and the law of constant composition. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.. The explanation of dalton's theory is as follows.

It can not be destroyed much less created. Atoms in chemical reactions can not be changed into other elements. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Diagram john dalton atomic model. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. According to john dalton the atom is like a solid ball.

Diagram john dalton atomic model. The element consists of particles that can not be divided again. The rutherford model also known as planetary model is a model which tried to. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. According to john dalton the atom is like a solid ball. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Diagram john dalton atomic model.. According to john dalton the atom is like a solid ball.

Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Dalton's atomic model sets up the building blocks for others to improve on. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. He defined an atom as the smallest indivisible particle. Though some of his conclusions were incorrect, his contributions were vital. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. 01.12.2014 · john dalton, the father of modern atomic theory. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. The element consists of particles that can not be divided again... Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat.
01.12.2014 · john dalton, the father of modern atomic theory.. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged.

According to john dalton the atom is like a solid ball. The element consists of particles that can not be divided again. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. The explanation of dalton's theory is as follows. Though some of his conclusions were incorrect, his contributions were vital. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Dalton based his theory on the law of conservation of mass and the law of constant composition.

Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. The explanation of dalton's theory is as follows. Although the concept of the atom dates back to the ideas of democritus, the english meteorologist and chemist john dalton formulated the first modern description of it as the fundamental building block of chemical structures. Though some of his conclusions were incorrect, his contributions were vital. Diagram john dalton atomic model.. The explanation of dalton's theory is as follows.

Dalton states that the substance consists of atoms that can not be divided again... In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads... Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat.

It can not be destroyed much less created. Dalton based his theory on the law of conservation of mass and the law of constant composition. Dalton's atomic model sets up the building blocks for others to improve on. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Dalton states that the substance consists of atoms that can not be divided again. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … He defined an atom as the smallest indivisible particle. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. The first part of his theory states that all matter is made of atoms, which are indivisible. Though some of his conclusions were incorrect, his contributions were vital.

Atoms in chemical reactions can not be changed into other elements. Dalton states that the substance consists of atoms that can not be divided again. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. The first part of his theory states that all matter is made of atoms, which are indivisible. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … It can not be destroyed much less created. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Diagram john dalton atomic model... In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.

He defined an atom as the smallest indivisible particle. The explanation of dalton's theory is as follows. In 1803, dalton orally presented his first list of relative atomic weights for a number of substances. 01.12.2014 · john dalton, the father of modern atomic theory. The element consists of particles that can not be divided again. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat.

The element consists of particles that can not be divided again. Dalton's atomic model sets up the building blocks for others to improve on. Diagram john dalton atomic model. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. The rutherford model also known as planetary model is a model which tried to. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Although the details of his diagrams were not always correct, the innovative principle of diagrammatically … Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time.

The element consists of particles that can not be divided again. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. According to john dalton the atom is like a solid ball. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. The first part of his theory states that all matter is made of atoms, which are indivisible.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. Though some of his conclusions were incorrect, his contributions were vital. The bohr atomic model sometimes known as the rutherford bohr atomic model was a major milestone in the development of modern atomic theory. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. It can not be destroyed much less created. He defined an atom as the smallest indivisible particle. Dieser artikel gehört zu unserer serie atommodelle der chemie, in der wir verschiedene atommodelle beschreiben. The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads.. Dalton's atomic model sets up the building blocks for others to improve on.

The history of the study of the atomic nature of matter illustrates the thinking process that goes on in the philosophers and scientists heads. Diagram john dalton atomic model. The first part of his theory states that all matter is made of atoms, which are indivisible. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. It can not be destroyed much less created.. The first part of his theory states that all matter is made of atoms, which are indivisible.

Dalton's atomic model sets up the building blocks for others to improve on. It can not be destroyed much less created.

Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. Though some of his conclusions were incorrect, his contributions were vital.. The element consists of particles that can not be divided again.

The first part of his theory states that all matter is made of atoms, which are indivisible... Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. Diagram john dalton atomic model. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties.

Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft... Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of antoine lavoisier and joseph proust. Though we know today that they can be further divided into protons, neutrons, and electrons, his explanation was revolutionary for that period of time. 06.06.2017 · dalton created symbols to represent the different types of atoms, and used them to draw diagrams to represent how he thought atoms were arranged. 01.12.2014 · john dalton, the father of modern atomic theory. Dalton states that the substance consists of atoms that can not be divided again. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft. He defined an atom as the smallest indivisible particle. It can not be destroyed much less created. Atoms in chemical reactions can not be changed into other elements. 01.12.2014 · john dalton, the father of modern atomic theory.

In 1803, dalton orally presented his first list of relative atomic weights for a number of substances.. Though some of his conclusions were incorrect, his contributions were vital.. He defined an atom as the smallest indivisible particle.

The first part of his theory states that all matter is made of atoms, which are indivisible. Though some of his conclusions were incorrect, his contributions were vital. Dalton's atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. The explanation of dalton's theory is as follows. Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat. Es wurde 1808 von john dalton veröffentlicht und entspricht natürlich nicht mehr dem aktuellen stand der wissenschaft... Aber es gibt einen frühen einblick in die vorstellung, was man unter atomen und stoffen und deren aufbau zu verstehen hat.
